Oligonucleotides Clinical Trial Pipeline Analysis Demonstrates 280+ Key Companies at the Horizon Expected to Transform the Treatment Paradigm, Assesses DelveInsight
Oligonucleotides are short chains of nucleic acid molecules that can be used to treat or manage a variety of diseases. Oligonucleotides are being increasingly explored for a variety of therapeutic areas, such as rare diseases, cancer, infectious diseases, and genetic disorders. Technologies like antisense oligonucleotides (ASOs), small interfering RNAs (siRNAs), and aptamers are at the forefront of innovation.
New York, USA, June 17, 2025 (GLOBE NEWSWIRE) -- Oligonucleotides Clinical Trial Pipeline Analysis Demonstrates 280+ Key Companies at the Horizon Expected to Transform the Treatment Paradigm, Assesses DelveInsight
Oligonucleotides are short chains of nucleic acid molecules that can be used to treat or manage a variety of diseases. Oligonucleotides are being increasingly explored for a variety of therapeutic areas, such as rare diseases, cancer, infectious diseases, and genetic disorders. Technologies like antisense oligonucleotides (ASOs), small interfering RNAs (siRNAs), and aptamers are at the forefront of innovation.
DelveInsight’s 'Oligonucleotides Competitive Landscape 2025' report provides comprehensive global coverage of pipeline oligonucleotides in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the oligonucleotides pipeline domain.
Key Takeaways from the Oligonucleotides Pipeline Report
- DelveInsight’s oligonucleotides competitive report presents a robust market with over 280 active players developing more than 320 pipeline oligonucleotides.
- Key oligonucleotide companies such as Novartis, Astellas, Alnylam Pharmaceuticals, Ionis Pharmaceuticals, 4D Molecular Therapeutics, Avidity Biosciences, Suzhou Ribo Life Science, Amgen, ProQR Therapeutics, Stoke Therapeutics, MiNA Therapeutics, Sylentis, GSK, Silexion Therapeutics, Novo Nordisk A/S, Bio-Path Holdings, Sunhawk Vision Biotech, Isarna Therapeutics, Sirnaomics, Laboratoire Thea, Dyne Therapeutics, Vertex Pharmaceuticals, Korro Bio, Praxis-Precision-Medicines, Vico Therapeutics, BioMarin Pharmaceutical, TransCode Therapeutics, TME Therapeutics, ARTHEx Biotech, aptaTargets, CSPC Zhongnuo Pharmaceutical, ExoRNA Bioscience, Visirna Therapeutics, AiCuris, Comanche Biopharma, Tallac Therapeutics, and others are evaluating new oligonucleotides to improve the treatment landscape.
- Promising pipeline oligonucleotides such as Pelacarsen, Izervay, Nucresiran, ALN-6400, Zilganersen, 4D-150, Delpacibart Etedesiran, RBD1007, Olpasiran, GSK3228836, Sepofarsen, STK-001, MTL-CEBPA, SYL-1801, Loder, CDR132L, BP1001, SHJ002, ISTH0036, STP705, Ultevursen, DYNE-101, VX-670, KRRO 110, PRAX-222, VO659, BMN 351, TTX-MC138, TME151, ATX-01, ApTOLL, SYH2062, ER2001, VSA012, AIC468, CBP-4888, ALTA-002, and others are under different phases of oligonucleotide clinical trials.
Request a sample and discover the recent advances in oligonucleotide drugs @ Oligonucleotides Competitive Report
Oligonucleotides Overview
Oligonucleotides are short chains of nucleotides, composed of repeating units that include a ribose or deoxyribose sugar, nitrogenous bases, and a phosphate backbone. Their ability to selectively bind to complementary DNA or RNA strands allows them to form duplexes or, less commonly, more complex structures. This property makes oligonucleotides valuable tools for detecting specific nucleic acid sequences.
Typically ranging from 13 to 25 nucleotides in length, oligonucleotides can hybridize with target DNA or RNA. They are classified into several types, including antisense oligonucleotides (ASOs), small interfering RNAs (siRNAs), microRNAs (miRNAs), and aptamers. These molecules are being actively explored for their therapeutic potential in treating conditions such as neurodegenerative diseases, cancer, and rare diseases. Clinical trials are also evaluating their use in dermatological, gastrointestinal, and endocrine disorders.
Oligonucleotides influence gene expression through various mechanisms, such as RNA interference (RNAi), RNase H-mediated degradation, splicing modulation, inhibition of non-coding RNAs, gene activation, and programmable gene editing. As a result, oligonucleotide therapeutics, including ASOs, siRNAs, and splice-modulating oligonucleotides, are emerging as a powerful new class of drugs capable of targeting a broad range of genetic and non-genetic diseases.
However, their clinical success depends heavily on overcoming significant delivery challenges. Due to their high molecular weight, hydrophilicity, and negative charge, oligonucleotides face difficulty crossing cell membranes. They are also prone to degradation by nucleases, have limited tissue penetration, are quickly cleared by the kidneys, and often require targeted delivery to specific tissues.
Despite these hurdles, oligonucleotides have transformed both diagnostics and therapeutics thanks to their high specificity, programmable nature, and ease of synthesis. In diagnostics, they serve key roles as primers in PCR/qPCR, probes in assays like FISH, and functional elements in technologies such as microarrays and CRISPR-based platforms. Aptamers enhance imaging by targeting specific biomarkers, and chemical modifications can significantly improve their stability in biological environments.
Therapeutically, oligonucleotides include drugs like pegaptanib (aptamer), nusinersen (ASO), and patisiran (siRNA), which work by silencing or modulating gene expression. They also play a foundational role in mRNA and DNA vaccines, miRNA inhibitors, and gene editing tools like CRISPR. While challenges such as off-target effects and rapid systemic clearance remain, innovations like GalNAc conjugation and AI-driven design are advancing the field, enhancing specificity, delivery efficiency, and clinical effectiveness, and propelling the era of precision medicine forward.
Oligonucleotides Market Dynamics
The oligonucleotides market is experiencing significant growth, driven by advances in genomic research, increased prevalence of genetic disorders, and rising interest in personalized medicine. Key therapeutic modalities, including antisense oligonucleotides (ASOs), small interfering RNAs (siRNAs), and aptamers, are gaining traction due to their ability to target diseases at the genetic level. The success of RNA-based drugs like SPINRAZA (nusinersen) and ONPATTRO (patisiran) has validated the commercial potential of oligonucleotide therapeutics, spurring further investment and innovation in the space.
The market dynamics are shaped by several influential trends. Technological advancements in oligonucleotide synthesis and chemical modifications have improved stability, bioavailability, and target specificity, thereby expanding clinical applications. Concurrently, the adoption of automated synthesis platforms and solid-phase synthesis technologies has increased manufacturing efficiency and scalability. However, production remains complex and cost-intensive, especially for therapeutic-grade oligonucleotides, creating opportunities for specialized CDMOs that can meet stringent regulatory and quality standards.
Pharmaceutical and biotech companies are aggressively expanding their oligonucleotide-based therapeutic pipelines, targeting a broad spectrum of indications including rare genetic diseases, cancer, neurodegenerative disorders, and infectious diseases. The oncology segment, in particular, is attracting attention for oligonucleotide therapies due to their ability to silence oncogenes or modulate gene expression with high precision. Moreover, the regulatory environment is becoming more supportive, with agencies like the FDA and EMA providing clear guidance for oligonucleotide drug development, fast-track designations, and orphan drug incentives.
Despite its promise, the oligonucleotides market faces several challenges. These include delivery barriers, especially for intracellular targets, off-target effects, and immunogenicity concerns. Lipid nanoparticles (LNPs), conjugation strategies (e.g., GalNAc), and novel carrier systems are under active investigation to address these issues. Furthermore, intellectual property landscapes are becoming increasingly complex as more players enter the field, potentially leading to patent disputes and licensing complexities.
In conclusion, the oligonucleotides market is poised for sustained growth, driven by technological innovation, clinical success stories, and expanding therapeutic frontiers. As barriers to delivery and cost are gradually overcome, oligonucleotide therapeutics are expected to become a mainstay in precision medicine, especially in areas with unmet medical needs. Strategic partnerships, regulatory clarity, and continued investment in R&D will be crucial to fully unlocking the potential of this transformative modality.
To know more about oligonucleotides, visit @ Oligonucleotides Market Insights
Approved Oligonucleotides Drug Profile Analysis
LEQVIO: Novartis
LEQVIO (inclisiran) is a first-of-its-kind small interfering RNA (siRNA) therapy developed by Novartis that targets the mRNA of PCSK9 (proprotein convertase subtilisin/kexin type 9). Unlike traditional treatments, LEQVIO reduces the production of the PCSK9 protein in the liver, enhancing the liver’s ability to absorb and eliminate LDL-C from the bloodstream. In 2023, Japan’s Ministry of Health, Labour and Welfare (MHLW) approved LEQVIO for both familial and non-familial hypercholesterolemia, as well as for individuals at high risk of cardiovascular events. The treatment regimen involves an initial dose, a second injection after three months, and subsequent maintenance doses every six months. Clinical trials have shown that when used alongside statins, LEQVIO can lower LDL-C levels by around 50%.
IZERVAY: Astellas Pharma
Avacincaptad pegol, sold under the brand name IZERVAY, is an approved treatment specifically developed for geographic atrophy. It is an RNA aptamer chemically linked to a branched polyethylene glycol (PEG) molecule. The drug works by targeting and inhibiting complement factor C5, a critical element of the complement system involved in inflammation related to age-related macular degeneration (AMD). By preventing the conversion of C5 into its active forms (C5a and C5b), avacincaptad pegol helps reduce inflammation and slow GA progression. The FDA approved IZERVAY on August 4, 2023, for GA secondary to AMD. It is currently under evaluation by the European Medicines Agency and is also being studied for potential use in treating Stargardt disease.
Find out more about oligonucleotide drugs @ Oligonucleotide Analysis
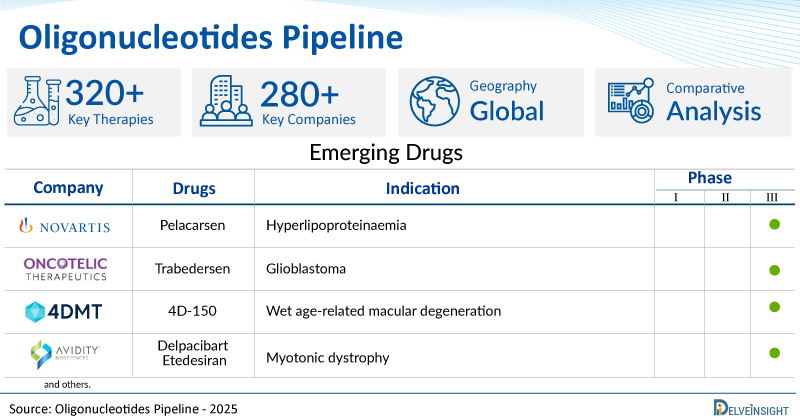
A snapshot of the Pipeline Oligonucleotides mentioned in the report:
| Drugs | Company | Phase | Indication |
| Pelacarsen | Novartis Pharmaceuticals | III | Hyperlipoproteinaemia |
| Trabedersen | Oncotelic | III | Glioblastoma |
| 4D-150 | 4D Molecular Therapeutics | III | Wet age-related macular degeneration |
| Delpacibart Etedesiran | Avidity Biosciences | III | Myotonic dystrophy |
| GSK3228836 | GSK | III | Chronic hepatitis B virus infection |
| WVE-N531 | Wave Life Sciences | II | Duchenne muscular dystrophy |
| ATX-01 | ARTHEx Biotech | II | Myotonic dystrophy Type I & II |
| TAC001 | Tallac Therapeutics | I/II | Solid tumors |
| ATB 301 | Autotelic Bio | I | Pancreatic cancer |
Learn more about the emerging oligonucleotides @ Oligonucleotides Clinical Trials
Key Developments in the Oligonucleotides Treatment Space
- In May 2025, Cure Rare Disease (CRD), announced that the U.S. Food and Drug Administration (FDA) has granted Orphan Drug Designation (ODD) to its investigational anti-sense Oligonucleotide therapeutic for the treatment of Spinocerebellar Ataxia (SCA), including Spinocerebellar Ataxia Type 3 (SCA3), a progressive and currently untreatable neurodegenerative disorder.
- In April 2025, Biogen Inc. announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track designation to BIIB080, an investigational antisense oligonucleotide (ASO) therapy targeting tau, for the treatment of Alzheimer’s disease.
- In March 2025, Korro Bio, Inc., a clinical-stage biopharmaceutical company focused on developing a new class of genetic medicines based on editing RNA for both rare and highly prevalent diseases, announced that the FDA had granted orphan drug designation to the investigational medicine KRRO-110 for the treatment of Alpha-1 Antitrypsin Deficiency (AATD).
- In February 2025, AusperBio Therapeutics, Inc. and Ausper Biopharma Co., Ltd. (together AusperBio), a clinical-stage biotechnology company, announced recent progress in the ongoing clinical development of its lead candidate AHB-137, an antisense oligonucleotide (ASO) therapeutic for functional cure of chronic Hepatitis B (CHB).
- In January 2025, Arrowhead Pharmaceuticals, Inc. announced that the US Food and Drug Administration (FDA) had accepted the New Drug Application (NDA) for investigational plozasiran for the treatment of familial chylomicronemia syndrome (FCS), a severe and rare genetic disease.
- In December 2024, Vir Biotechnology, Inc. announced that tobevibart and elebsiran have received U.S. Food and Drug Administration (FDA) Breakthrough Therapy designation and European Medicines Agency (EMA) Priority Medicines (PRIME) designation for the treatment of chronic hepatitis delta (CHD).
- In December 2024, the FDA granted breakthrough therapy designation to Stoke Therapeutics' investigational antisense agent STK-001 for the treatment of genetically confirmed Dravet syndrome (DS), a rare epilepsy disorder.
- In November 2024, Ionis Pharmaceuticals announced that the US Food and Drug Administration (FDA) had accepted for review the New Drug Application (NDA) for donidalorsen, an investigational RNA-targeted medicine for prophylaxis to prevent attacks of hereditary angioedema (HAE) in adult and pediatric patients 12 years of age and older. The FDA has set an action date of August 21, 2025, under the Prescription Drug User Fee Act (PDUFA).
- In October 2024, Ribocure Pharmaceuticals AB and Suzhou Ribo Life Science Ltd received authorization from the Swedish Medicinal Product Agency (MPA) to initiate a Phase II clinical trial in Sweden with the lipid-lowering siRNA drug RBD5044 that targets APOC3. The trial will evaluate efficacy and safety in patients with mixed dyslipidemia
- In September 2024, WVE-N531, an exon-skipping oligonucleotide developed by Wave Life Sciences for the treatment of Duchenne muscular dystrophy (DMD) in patients amenable to exon 53 skipping, received Orphan Drug Designation from the US Food and Drug Administration (FDA).
- In September 2024, NS Pharma, Inc., a subsidiary of Nippon Shinyaku Co., Ltd. (Nippon Shinyaku), announced that the Food and Drug Administration (FDA) had granted rare pediatric disease designation to NS050/NCNP-03, which is being developed for the treatment of Duchenne muscular dystrophy (Duchenne).
- In May 2024, Sunhawk Vision Biotech announced that it had received authorization from the US FDA to commence a Phase II clinical trial for myopia control in children.
- In May 2024, Imvax, Inc., announced the completion of enrollment in its randomized, multicenter, double-blind, placebo-controlled Phase IIb clinical trial of IGV-001 in patients with newly diagnosed glioblastoma (ndGBM).
Scope of the Oligonucleotides Pipeline Report
- Coverage: Global
- Key Oligonucleotides Companies: Novartis, Astellas, Alnylam Pharmaceuticals, Ionis Pharmaceuticals, 4D Molecular Therapeutics, Avidity Biosciences, Suzhou Ribo Life Science, Amgen, ProQR Therapeutics, Stoke Therapeutics, MiNA Therapeutics, Sylentis, GSK, Silexion Therapeutics, Novo Nordisk A/S, Bio-Path Holdings, Sunhawk Vision Biotech, Isarna Therapeutics, Sirnaomics, Laboratoire Thea, Dyne Therapeutics, Vertex Pharmaceuticals, Korro Bio, Praxis-Precision-Medicines, Vico Therapeutics, BioMarin Pharmaceutical, TransCode Therapeutics, TME Therapeutics, ARTHEx Biotech, aptaTargets, CSPC Zhongnuo Pharmaceutical, ExoRNA Bioscience, Visirna Therapeutics, AiCuris, Comanche Biopharma, Tallac Therapeutics and others.
- Key Oligonucleotides in Pipeline: Pelacarsen, Izervay, Nucresiran, ALN-6400, Zilganersen, 4D-150, Delpacibart Etedesiran, RBD1007, Olpasiran, GSK3228836, Sepofarsen, STK-001, MTL-CEBPA, SYL-1801, Loder, CDR132L, BP1001, SHJ002, ISTH0036, STP705, Ultevursen, DYNE-101, VX-670, KRRO 110, PRAX-222, VO659, BMN 351, TTX-MC138, TME151, ATX-01, ApTOLL, SYH2062, ER2001, VSA012, AIC468, CBP-4888, ALTA-002 and others are under different phases of oligonucleotide clinical trials.
Dive deep into rich insights for new oligonucleotide treatments, visit @ Oligonucleotides Drugs
Table of Contents
| 1. | Oligonucleotides Pipeline Report Introduction |
| 2. | Oligonucleotides Pipeline Report Executive Summary |
| 3. | Oligonucleotides Pipeline: Overview |
| 4. | Oligonucleotides Marketed Drugs |
| 4.1. | LEQVIO: Novartis Pharmaceuticals |
| 5. | Oligonucleotides Clinical Trial Therapeutics |
| 6. | Oligonucleotides Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Oligonucleotides Pipeline: Late-Stage Products (Phase III) |
| 7.1. | Pelacarsen: Novartis Pharmaceuticals |
| 8. | Oligonucleotides Pipeline: Mid-Stage Products (Phase II) |
| 8.1. | Trabedersen: Oncotelic |
| 9. | Oligonucleotides Pipeline: Early-Stage Products (Phase I) |
| 9.1. | ATB 301: Autotelic Bio |
| 10. | Oligonucleotides Pipeline: Preclinical and Discovery Stage Products |
| 11. | Oligonucleotides Pipeline Therapeutics Assessment |
| 12. | Inactive Products in the Oligonucleotides Pipeline |
| 13. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 14. | Unmet Needs |
| 15. | Oligonucleotides Market Drivers and Barriers |
| 16. | Appendix |
For further information on the oligonucleotides pipeline therapeutics, reach out @ Oligonucleotides Therapeutics
Related Reports
Antisense Oligonucleotide Therapeutics Pipeline
Antisense Oligonucleotide Therapeutics Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key antisense oligonucleotide therapeutics companies, including Ionis Pharmaceuticals, Secarna Pharmaceuticals, Aro Biotherapeutics, NeuBase Therapeutics, Bio-Path Holdings, Inc., Scopus Biopharma, Dyne Therapeutics, CAMP4 Therapeutics, Pulmotect, GeneTx Biotherapeutics, Aligos Therapeutics, WaVe Life Sciences, among others.
Oligonucleotide Synthesis Market
Oligonucleotide Synthesis Market Insight, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of market trends, market drivers, market barriers, and key oligonucleotide synthesis companies, including Thermo Fisher Scientific Inc., Agilent Technologies, Merck KGaA, Bio-Synthesis Inc., Ajinomoto Bio-Pharma Services, CordenPharma, Creative Biolabs, Ella Biotech, Eurofins Genomics, Future Synthesis, Integrated DNA Technologies, Kaneka Eurogentec, LGC Biosearch Technologies, Microsynth, Nitto Avecia, Ribo Biotechnology, STA Pharmaceutical, Sumitomo Chemical, TriLink Biotechnologies, Sarepta Therapeutics, among others.
RNA Interference Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key RNA interference companies, including Silence Therapeutics, Janssen Research & Development, Eli Lilly and Company, Arrowhead Pharmaceuticals, Sylentis, Sirnaomics, Dicerna Pharmaceuticals, Suzhou Ribo Life Science, Alnylam Pharmaceuticals, Suzhou Ribo Life Science, Vir Biotechnology, Arbutus Biopharma, Silenseed, OliX Pharmaceuticals, Bio-Path Holdings, among others.
Global Messenger RNA (mRNA)-based Vaccines and Therapeutics Market
Global Messenger RNA (mRNA)-based Vaccines and Therapeutics Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key global messenger RNA -based vaccines and therapeutics companies, including Moderna, Inc., BioNTech SE, CureVac N.V., Arcturus Therapeutics, Translate Bio, Inc., GSK, among others.
mRNA Vaccines and Therapeutics Market
mRNA Vaccines and Therapeutics Market Insights, Competitive Landscape, and Market Forecast – 2032 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key mRNA vaccines and therapeutics companies, including Pfizer Inc., BioNTech SE, Moderna, Inc., Gennova Biopharmaceuticals Limited, GSK plc., Daiichi Sankyo, Arcturus, Boehringer Ingelheim International GmbH, Ethris GmbH, CureVac SE, AIM Vaccine Corporation, Charoen Pokphand Group, Argos Therapeutics Inc., Sanofi, Kernal Biologics Inc, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
